Research & Development | Clinical Testing | Commercial Partnership

Our Groundbreaking Technology
EpiSign™ technology utilizes artificial intelligence to analyze genome-wide DNA methylation profiles, known as episignatures, to diagnose rare disorders.
About Episign™
EpiSign™ is a world-leading DNA methylation episignature testing service that showcases the enormous potential of advanced clinical diagnostics.
Based on the global research effort coming from the world’s largest rare disease Episign Knowledge Database, EpiSign™ helps patients get a diagnosis that cannot be provided by standard genetic testing.
EpiSign™ Complete
EpiSign™ Complete is the first clinical assay validated to detect unique epigenetic signatures and methylation abnormalities for more than 113 recognized genetic conditions.
EpiSign™ Target
EpiSign™ Target is a targeted review of methylation data intended to resolve variants of uncertain clinical significance in genes with a known epigenetic signature.
EpiSign™ Single
Learn more about getting a test
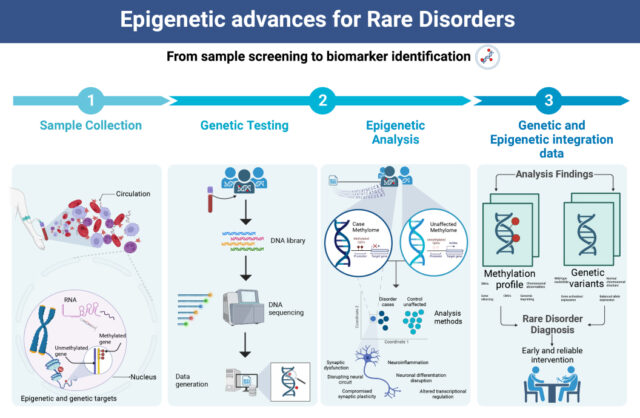
How EpiSign™ Works
EpiSign™ leverages cost-effective microarray technology for assessment of genome-wide DNA methylation patterns using the EpiSign™ proprietary software to enable a diagnosis for patients with rare diseases beyond the capabilities of standard genomic testing.
Utilizing peripheral blood, patients’ methylation data is screened to determine if they possess a similar methylation profile, or episignature, to a defined episignature associated with a specific rare disorder within the EpiSign™ Knowledge Database. Episignatures provide highly sensitive and specific molecular biomarkers.
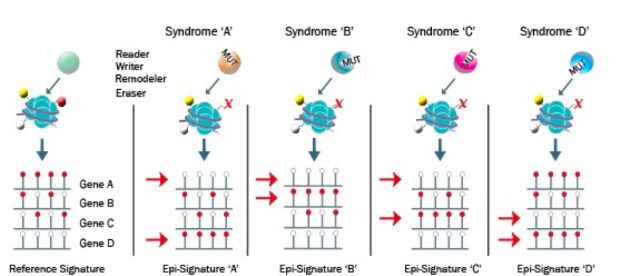
What is an episignature?
An episignature is a unique and reproducible DNA methylation profile, or biomarker, associated with a specific rare disorder including genetic and environmental exposure conditions.
EpiSign™ Knowledge Database
The world’s largest epigenetic database for rare diseases containing thousands of reference samples, including a growing list of genetic conditions.
When EpiSign™ is Used
Screening patients with complex or ambiguous presentations.
Reclassification of variants of uncertain significance (VUS).
Detecting exposure to teratogens during pregnancy.
Investigating reduced penetrance or expressivity of a genetic variant.
Assessing for imprinting defects or gene silencing due to promoter hypermethylation.
Confirm clinical diagnosis when genetic variant is unknown or undetected.
EpiSign™ in Action
The EpiSign™ Clinical Testing Network emphasizes the test’s value as an adjunct to traditional DNA sequence analysis, particularly in rare diseases, showcasing a remarkable 18.7% and 32.4% diagnostic yield in comprehensive and targeted tests, respectively.
EpiSign™ Around the Globe

Our Discovery Network
We collaborate with more than 100 institutions in over 20 countries in a global research effort to expand the impact of EpiSign™ worldwide.

EpiSign™-CAN Clinical Trial
The first national clinical trial of its kind with the ultimate goal of measuring the clinical and health systems impact of the EpiSign™ test within Canada.

EpiSign™ International Clinical Trial
An international clinical trial that involves institutions across 15 countries for validation of integrated genomic and epigenomic hardware and automated end user EpiSign software.

